Z6_EL9QHKG10GPM20IJGE61AK10O0
Z7_P86CH0O0I0RF10IB06HRVQ0087
 S2K Commerce - Products Dropdown
S2K Commerce - Products Dropdown
Z7_60E2I18209KBD06G17A9D51006
 Web Content Viewer
Web Content Viewer
Z7_EL9QHKG10GPM20IJGE61AK10S5
 S2K Commerce - Order Entry
S2K Commerce - Order Entry
You must be logged in to order the below items. Log in to order
RPR Control Cards 18 mm circle controls R, RM, NR spots. Packaging: 10/Pack.
Item#:
191912
Manufacturer Item:
276709
Manufacturer:
BD DIAGNOSTICS
UNSPSC:
41116128
1,000 mL vacuum bottle with drainage line. Packaging: 10/Case.
Item#:
192595
Manufacturer Item:
50-7210
Manufacturer:
CAREFUSION 2200
UNSPSC:
42144101
Home Kit Mailer Package, each kit contains a mailer, 2 collection papers and return form instructions, 2-30°C. Hemosure Fecal Occult Blood Tests employ a unique combination of monoclonal and polyclonal antibodies to detect only human blood in stool. The test can be performed by health care providers in hospitals, labs and private practices. Test results can be quickly and accurately read in less than 5 minutes. The new technology has greatly improved specificity, sensitivity, accuracy and cost-effectiveness of fecal occult blood tests. Packaging: 50/Box.
Item#:
192740
Manufacturer Item:
T1-CM50
Manufacturer:
HEMOSURE, INC.
UNSPSC:
41116168
CLEARVIEW HCG DIPSTICK KIT, 25MIU/ML. Packaging: 50/Box.
Item#:
195751
Manufacturer Item:
92211
Manufacturer:
ALERE NORTH AMERICA, LLC.
UNSPSC:
41116136
Urine 6-Panel Drug Test, Cassette CLIA Waived Accutest. Packaging: 25/Box.
Item#:
196842
Manufacturer Item:
DS56
Manufacturer:
JANT PHARMACAL CORPORATION
UNSPSC:
41116146
COULTER S-CAL Calibrator Kit, 2 x 4.2 mL. Packaging: 1/Each.
Item#:
197062
Manufacturer Item:
624519
Manufacturer:
BECKMAN COULTER, INC.
UNSPSC:
41116122
COULTER LATRON Control, 5 x 16 mL. Packaging: 1/Kit/Each.
Item#:
197065
Manufacturer Item:
7546914
Manufacturer:
BECKMAN COULTER, INC.
UNSPSC:
41116122
COULTER LATRON Primer, 5 x 16 mL. Packaging: 1/Kit/Each.
Item#:
197066
Manufacturer Item:
7546915
Manufacturer:
BECKMAN COULTER, INC.
UNSPSC:
41116121
COULTER 5C Cell Control, Tri-Pack, 12 x 3.3 mL. Packaging: 1/Kit/Each.
Item#:
197071
Manufacturer Item:
7547116
Manufacturer:
BECKMAN COULTER, INC.
UNSPSC:
41116122
COULTER LIN-C Linearity Controls, 11 x 3.3 mL. Packaging: 1/Kit/Each.
Item#:
197073
Manufacturer Item:
723503
Manufacturer:
BECKMAN COULTER, INC.
UNSPSC:
41116122
COULTER Retic-C Cell Control (Gen S, LH), 9 x 3.3 mL. Packaging: 1/Kit/Each.
Item#:
197074
Manufacturer Item:
7547125
Manufacturer:
BECKMAN COULTER, INC.
UNSPSC:
41116122
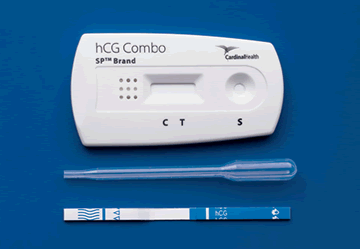
SP Urine Cassette Test. SP hCG tests are rapid, one-step tests for the qualitative detection of human chorionic gonadotropin in urine. The simple, one-step procedure provides results in 3 minutes for urine. Built-in test controls ensure accuracy. Kit contains test cassettes with disposable specimen droppers and easy-to-follow directional inserts. Store at room temperature or refrigerate at 2° to 30°C. Packaging: 1/Kit/Box.
Item#:
198496
Manufacturer Item:
B1077-22
Manufacturer:
CARDINAL HEALTHCARE CORPORATON
UNSPSC:
41116205
Item#:
198930
Manufacturer Item:
852-010
Manufacturer:
ALERE NORTH AMERICA, LLC.
UNSPSC:
41116139
Sure-Vue Signature Mono Test kit. Sensitivity: 100%; Specificity: 100.0%; Test time: five min.; Sample types: Serum, plasma, whole blood. Packaging: 25/Packaging.
Item#:
198951
Manufacturer Item:
23-200-275
Manufacturer:
THERMO FISHER SCIENTIFIC
UNSPSC:
41116205
Item#:
198955
Manufacturer Item:
852-010
Manufacturer:
ALERE NORTH AMERICA, LLC.
UNSPSC:
41116139
Test Kit Binaxnow RSV Swab Control, Neonatal & Pediatric. Packaging: 10/Kits/Pack.
Item#:
198956
Manufacturer Item:
430-080
Manufacturer:
ALERE NORTH AMERICA, LLC.
UNSPSC:
41116145
PHENOL 25% 2OZ ORM-D. Packaging: 2-oz/Each.
Veritor System for Rapid Detection of Flu A+B BD Veritor System (CLIA-waived) for Rapid Detection of Flu A+B is a rapid chromatographic immunoassay for the direct and qualitative detection of influenza A and B viral nucleoprotein antigens from nasal and nasopharyngeal swabs of symptomatic patients. UOM 1 Case/30.
Item#:
201503
Manufacturer Item:
256045
Manufacturer:
BD DIAGNOSTICS
UNSPSC:
41116144
ACCUTEST Value+ Urine Pregnancy Test Device (CLIA Waived). Rapid test for the detection of human chorionic gonadotropin (hCG) in urine specimens. This test is used to obtain a visual, qualitative result and is intended for professional and laboratory use. Results in 5 minutes or less.Easy to use cassette format. One-step test; no reagents needed. UOM: BX/25
Item#:
202746
Manufacturer Item:
PF853
Manufacturer:
JANT PHARMACAL CORPORATION
UNSPSC:
41116205
ImmunoCard STAT!® Rotavirus Rapid test for the detection of rotavirus antigen in human stool. Simple procedure requires no filtration and produces results in approximately 10 minutes. Trusted, accurate performance with clearly defined test bands for easy interpretation. Shelf Life from date of manufacture-18 Months. Assay Format-Test Card. Results Interpretation Positive Test Results: Visual detection of red/purple Test and Control lines. Negative Test Results: Visual detection of red/purple Control line, No red/purple Test Line present. Invalid Test Results: No visual detection of red/purple Control line, with or without a visually detectable red/purple Test line. UOM: 30 Determinations/BX.
Item#:
203218
Manufacturer Item:
750030
Manufacturer:
MERIDIAN BIOSCIENCE, INC.
UNSPSC:
41116168
Fisher HealthCare Sure-Vue RPR Test Kit (Rapid Plasma Reagent). Includes: Reagent, prediluted positive and negative controls, dispensing pipets, needle, plastic dispensing vial, disposable slides, and package insert. Detects "reagin" present in Serum of syphilitic patients. Test results in eight minutes. Positive results yield a flocculation of the antigen; negative results yield no reaction and a gray homogeneous suspension, or a button of non-aggregating carbon particles in the center of the circle. For in vitro diagnostic use. UOM: 100/PK.
Item#:
203392
Manufacturer Item:
23-038-009
Manufacturer:
THERMO FISHER SCIENTIFIC
UNSPSC:
41116129
Alco Screen 02 Salvie Alcohol Test for 0 Tolerance Testing. Alco-Screen® 02 is a simple one-step saliva screening test that is minimally invasive and provides results within 4 minutes. Simply wet the test pad with saliva; development of a line on the test pad within 4 minutes indicates a blood alcohol concentration exceeding 0.02%. UOM: 24/BX, 12BX/CS.
Item#:
204796
Manufacturer Item:
56288
Manufacturer:
CHEMATICS, INC.
UNSPSC:
41116146
CUP URINE 100ML STERILE ***DISC BY MFG-SEE S#377487***
This item has been discontinued and replaced by item
377487
VACUETTE® Urine Cup w/ Integrated Transfer Device 100 ml, Beaker Material: Polyethylene Therephthalate. CE Mark: Yes. Storage: 4 - 25°C. Indication: For Collection of Urine Specimens. UOM: 200/CS.
Item#:
205444
Manufacturer Item:
724310
Manufacturer:
GREINER BIO-ONE NORTH AMER,INC
Item#:
206368
Manufacturer Item:
269062
Manufacturer:
PROPPER MANUFACTURING CO.,INC.
UNSPSC:
42281810
Hemosure One-Step Immunological Fecal Occult Blood (IFOB) Test Home Kit Mailer. Kit includes: 25 Fecal Occult Blood Test Cassettes; 25 Home Kit Mailer Kits/Package: (Each kit includes 1 Collection Tube, 2 collection papers, patient instructions and return envelope). 24 month shelf life (expiration date printed on packaging). Hemosure One-Step Immunological Fecal Occult Blood Test is a qualitative, sandwich dye conjugate immunoassay and employs a unique combination of monoclonal and polyclonal antibodies to selectively identify in human feces, as an aid in diagnosis of fecal occult blood, with a high degree of sensitivity. In less than five minutes, elevated levels of human hemoglobin as low as 0.05ugHb/ml can be detected and positive results for high levels of hemoglobin can be seen in the test as early as one or two minutes. UOM: 25 prepacked home kit mailers (1 collection tube per mailer).
Item#:
206883
Manufacturer Item:
PREPACK-CM25
Manufacturer:
HEMOSURE, INC.
UNSPSC:
41116168