Z6_EL9QHKG10GPM20IJGE61AK10O0
Z7_P86CH0O0I0RF10IB06HRVQ0087
 S2K Commerce - Products Dropdown
S2K Commerce - Products Dropdown
Z7_60E2I18209KBD06G17A9D51006
 Web Content Viewer
Web Content Viewer
Z7_EL9QHKG10GPM20IJGE61AK10S5
 S2K Commerce - Order Entry
S2K Commerce - Order Entry
KIT RSV FOR VERITOR CLIA WAIVED
Item #:
223383
Manufacturer Item: 256038
Manufacturer:
BD DIAGNOSTICS
UNSPSC: 41116144
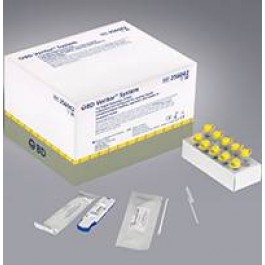
BD Veritor System - CLIA-waived for Rapid Detection of Respiratory Syncytial Virus (RSV). The BD Veritor System for Rapid Detection of Respiratory Syncytial Virus (RSV) is a chromatographic immunoassay with an instrumented read for the direct and qualitative detection of RSV fusion protein from a direct nasopharyngeal swab from patients suspected of having a viral respiratory infection. This test is intended for in vitro diagnostic use to aid in the diagnosis of RSV infections in infants and pediatric patients under the age of 6 years. Negative results do not preclude RSV infection and should not be used as the sole basis for treatment or for other management decisions. A negative test is presumptive. It is recommended that negative test results be confirmed by viral cell culture or an alternative method, such as a FDA-cleared molecular assay. The test is intended for professional and laboratory use. It is to be used in conjunction with the BD Veritor System Reader. UOM: 30/BX.







